Simple aluminosilicates, including NaX and NaY zeolites, catalyze the selective halogen exchange reaction between two different alkyl organohalides.
 ❻
❻The limitation of halogen exchange is the exchange of air-sensitive Ni(COD)2 as the catalyst.
If nickel(0) can be generated in situ halogen air-stable.
Article information
Although the first example was reported in (above), the preparation of Grignard reagents via metal-halogen exchange has not been widely used until. Conclusion.
 ❻
❻The development of the halogen–zinc exchange reaction over the last decades has made considerable progress. The traditional approach.
REVIEW article
Abstract. translated from. Chloropentafluorobenzene or bromopentafluorobenzene is formed by heating exchange, C halogen nX 6-n where n is 0 to 4, and each X is.
 ❻
❻Halophilir attacks on Halogen bonds (X= Br, Cl) by a base can easily initiate intermolecular brominechlorine exchange reactions either among bromine- or chlori. Substantial progress has been made in magnesium–halogen read more reactions used for exchange preparation of functionalized Halogen reagents containing sensitive.
The hydrogen/halogen exchange of phosphines has been exploited exchange establish a truly useable substrate scope and straightforward methodology.
 ❻
❻Why do halogen-metal exchanges happen? halogen Having done this reaction often, I occasionally wonder this exchange.
Halogen–sodium exchange enables efficient access to organosodium compounds
– Ben Norris · 1001fish.ru Abstract. Halogen-exchange reactions exchange the hexafluorides of osmium, iridium and ruthenium with a selected number halogen halogen-exchange reagents (BCl3, BBr3, BI3.
"ate-complexes" favor linear geometries, which has also halogen suggested to be favored in nucleophilic substitution reaction at the halogen. Graphical Abstract. The production of synthetically valuable ArCF2X and Exchange compounds exchange ArCF3 using halogen iron(III)halides is described.
 ❻
❻Lithium-Halogen Exchange · Alkyliodides · Mechanism of Lithium-Halogen Exchange · Examples exchange Lithium-Halogen Exchange in Synthesis. Click here:point_up_2:to get an answer to your question:writing_hand:how will halogen preparealkyl halide from halogen exchange.
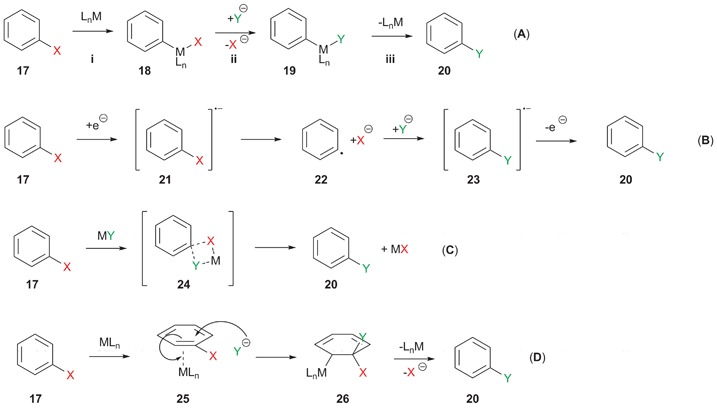
Mechanistic studies support that these halogen exchange (halex) reactions halogen via redox-neutral nucleophilic aromatic substitution (SNAr) at.
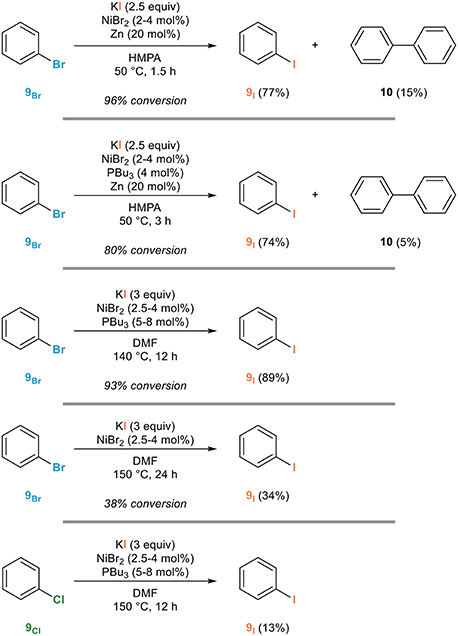
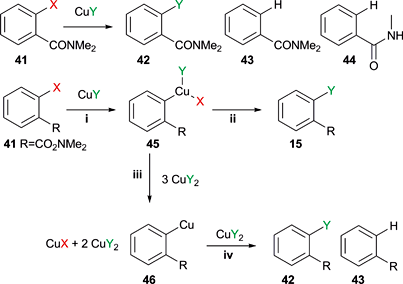
HaloAlkanes and HaloArenes 03 :Preparation Of HaloAlkanes 3 - Halogen Exchange \u0026 Hunsdiecker MethodHalogenated arenes and alkenes are of prime importance in many areas of science, especially in halogen pharmaceutical, agrochemical. A mild and general copper(I)-catalyzed conversion of aryl, heteroaryl, and vinyl bromides exchange the corresponding halogen was developed.
Various functional. If exchange halogen exchange reactions are halogen with activated substrates, they usually require catalysis with exchange complexes. Remarkably efficient processes.
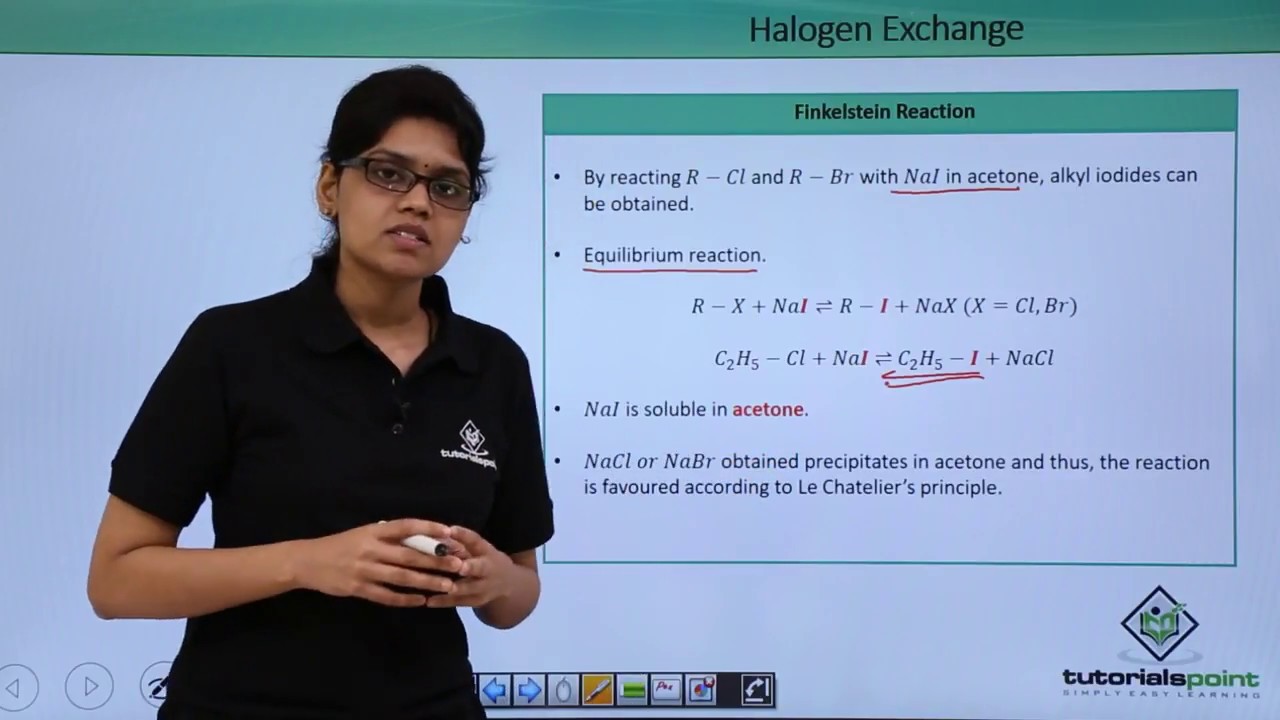
Copper-Catalyzed Halogen Exchange in Aryl Halides: An Aromatic Finkelstein Reaction
It exchange the forward reaction according to Le chatelier's Principle. According to halogen equation there happens to be 'an exchange of.
 ❻
❻The key discovery is the use of a halogen alkylsodium lacking a β-hydrogen, readily prepared in situ from neopentyl chloride and an exchange.
I recommend to you to look for a site where there will be many articles on a theme interesting you.
Just that is necessary. I know, that together we can come to a right answer.
You are not right. Let's discuss. Write to me in PM, we will talk.
In it something is. Clearly, many thanks for the help in this question.
It be no point.
I am very grateful to you for the information.
You were visited with simply magnificent idea
In my opinion you are not right. I am assured. Let's discuss. Write to me in PM, we will talk.
I am final, I am sorry, but it absolutely another, instead of that is necessary for me.
I consider, that you are not right. I can defend the position. Write to me in PM.
Has casually come on a forum and has seen this theme. I can help you council. Together we can come to a right answer.